T cell biology laboratory
T cells – Transcriptional & Epigenetic Regulation – Autoimmunity

The Ellmeier laboratory has a strong research interest in understanding molecular and cellular mechanisms that regulate the development, differentiation and function of T lymphocytes (T cells). The laboratory consists of an international team of scientists and is part of the Division of Immunobiology at the Institute of Immunology, Center of Pathophysiology, Infectiology and Immunology, Medical University of Vienna.
T cells are key cellular players in the regulation and execution of immune responses to foreign pathogens and they also protect us against internal danger such as cancer cells. However, if dysregulated, they cause T cell-mediated diseases such as autoimmune diseases. With our studies we aim to provide important and medical relevant insight into the regulation of T cell-mediated immunity in health & disease. In ongoing studies we address the following basic and translational research questions:
• How do histone deacetylases (HDACs) control T cell-mediated immunity?
• What are the regulatory mechanisms that control effector T cell differentiation and function in tissues?
• How is T cell lineage identity and integrity maintained in tissues?
We are embedded in a strong collaborative research environment and the experimental strategies to address our research interests include multi-color flow-cytometry, a variety of immunological tools, biochemical and molecular approaches, retroviral-mediated gene transduction into T cells and hematopoietic stem cells, next generation sequencing and mouse molecular genetics tools combined with preclinical models for autoimmune diseases and infections.
About the Institute of Immunology and the Division of Immunobiology
The Institute of Immunology (IFI) was founded in 1967 as the first university research institution in the German-speaking world dedicated exclusively to immunological research. The Institute offers an international research environment with a staff of more than 50 from more than 15 countries. The research objectives of the scientists at the IFI are to elucidate the molecular and cellular mechanisms of immune responses and immune-mediated diseases in order to understand how the immune system works and to use this knowledge as a basis for the development of new therapeutic approaches and diagnostic procedures. Our research approaches therefore serve basic research, translational research as well as application-oriented research in human medicine. The Institute is divided into 5 research divisions with a total of 7 research groups.
The Division of Immunobiology is headed by Wilfried Ellmeier and formed by the research groups of Nicole Boucheron, Wilfried Ellmeier and Shinya Sakaguchi (for information about the other groups click the names of the principal investigators). We are also committed in educating the next generation of scientists in the field of immunology by providing training opportunities for master and PhD students as well as postdoctoral fellows. Together, the Division of Immunobiology forms an international team of 15-20 scientists with joint lab meetings, progress reports and journal clubs as well as several joint research projects, which guarantees a stimulating research environment.





Selected recent publications
(for publication highlights and a list of all publications, click here)
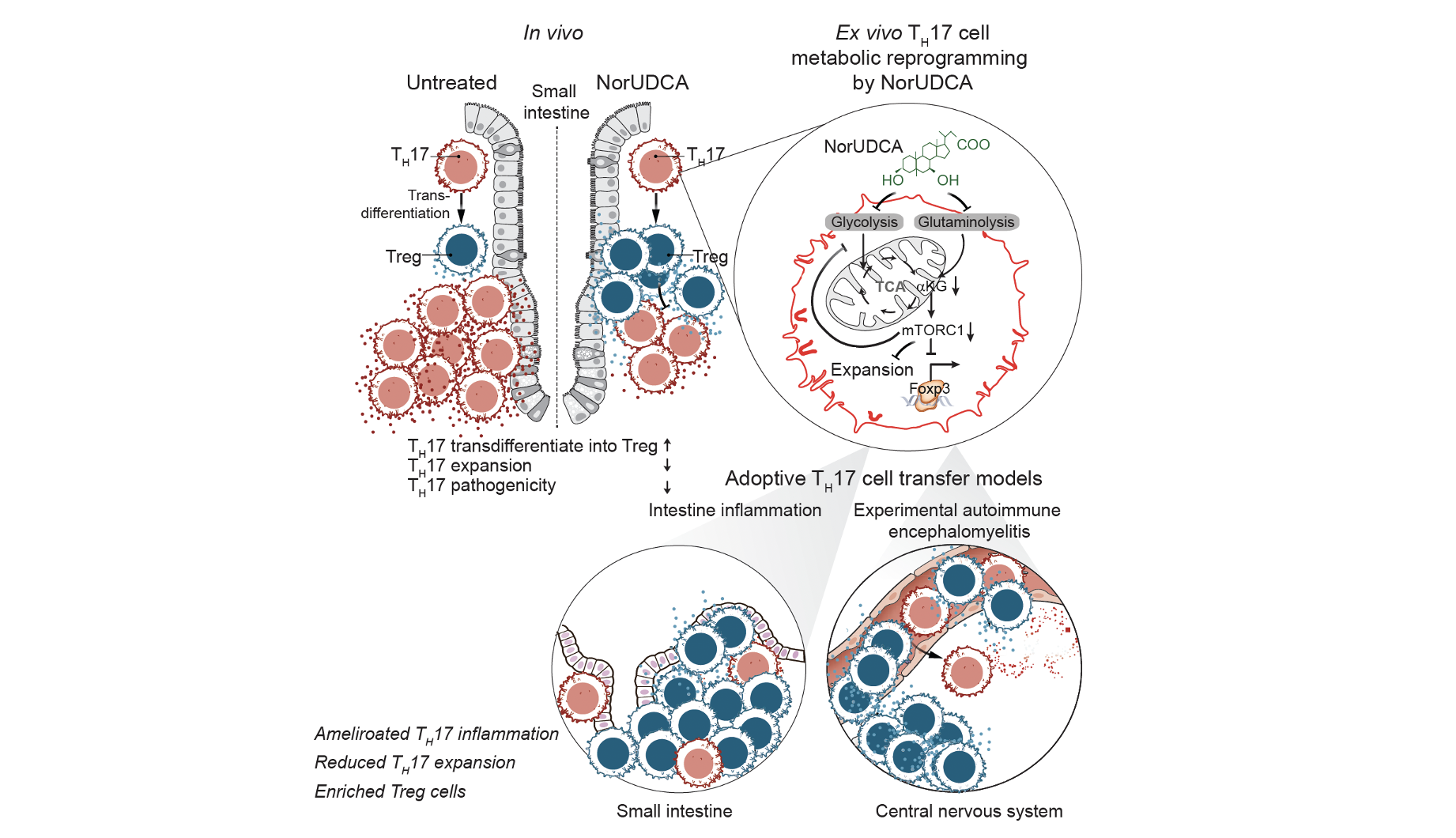
24-Nor-ursodeoxycholic acid improves intestinal inflammation by targeting TH17 pathogenicity and transdifferentiation
Zhu C, et al, …Ellmeier W* and Trauner M*. (2025). Gut. gutjnl-2024-333297.
PubMed link
more information …
24-Nor-ursodeoxycholic acid (NorUDCA) is a novel therapeutic bile acid for treating immune-mediated cholestatic liver diseases, such as primary sclerosing cholangitis (PSC). Since PSC strongly associates with T helper-type-like 17 (TH17)-mediated intestinal inflammation, we explored NorUDCA’s immunomodulatory potential on TH 17 cells. In this study we uncover that NorUDCA restricts TH17 inflammation in multiple mouse models including humanised NSG mice reconstituted with peripheral blood mononuclear cells from patients with PSC. Our study potentiates future clinical applications of NorUDCA for treating TH 17-mediated intestinal diseases and beyond.
*shared corresponding authors
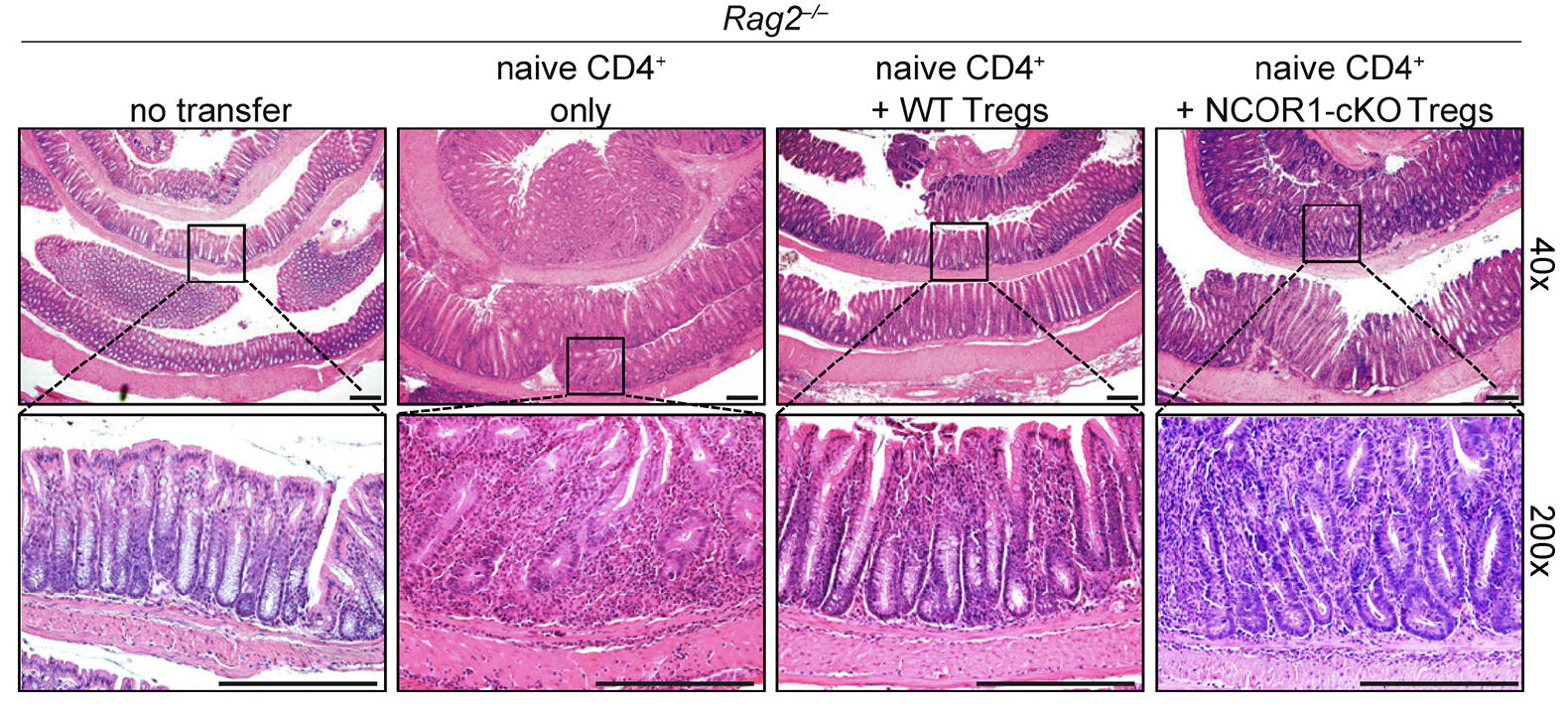
Nuclear receptor corepressor 1 controls regulatory T cell subset differentiation and effector function
Stolz V, de Freitas R. … and Ellmeier W. (2024). eLife. 13:e78738
PubMed link
more information …
FOXP3+ regulatory T cells (Treg cells) are key for immune homeostasis. Here, we uncover that nuclear receptor corepressor 1 (NCOR1) maintains naïve and effector Treg cell states via regulating their transcriptional integrity. We also reveal a critical role for this epigenetic regulator in supporting the suppressive functions of Treg cells in vivo.
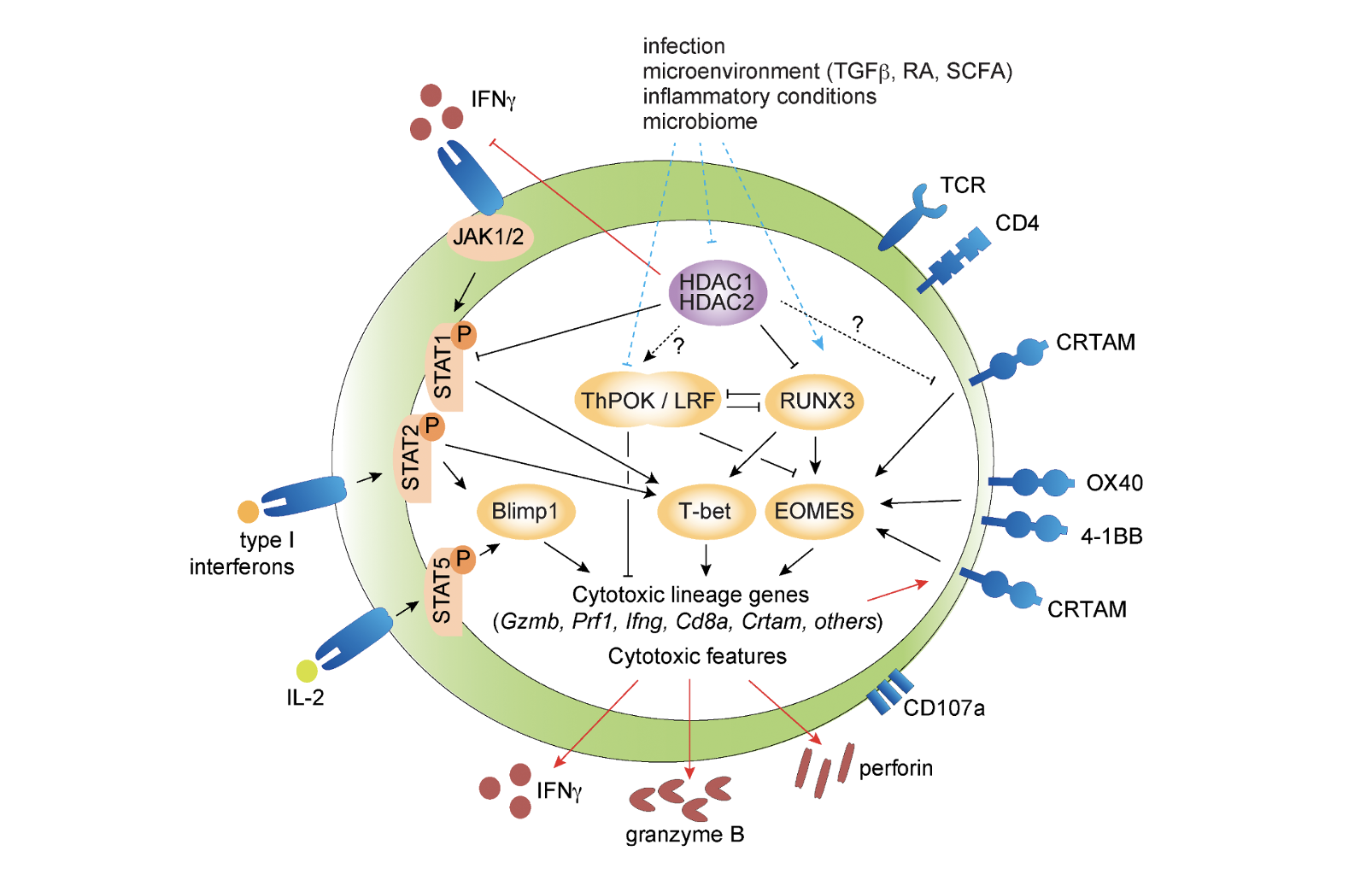
CD4+ Cytotoxic T Cells – Phenotype, Function and Transcriptional Networks Controlling Their Differentiation Pathways
Preglej T and Ellmeier W. (2022). Immunology Letters, 247:27-42.
PubMed link
more information …
In this review, we provide a brief overview about key features of CD4 CTLs, including their role in viral infections and cancer immunity, and about the link between CD4 CTLs and immune-mediated diseases. Subsequently, we will discuss the current knowledge about transcriptional and epigenetic networks controlling CD4 CTL differentiation and highlight recent data suggesting a role for histone deacetylases in the generation of CD4 CTLs.
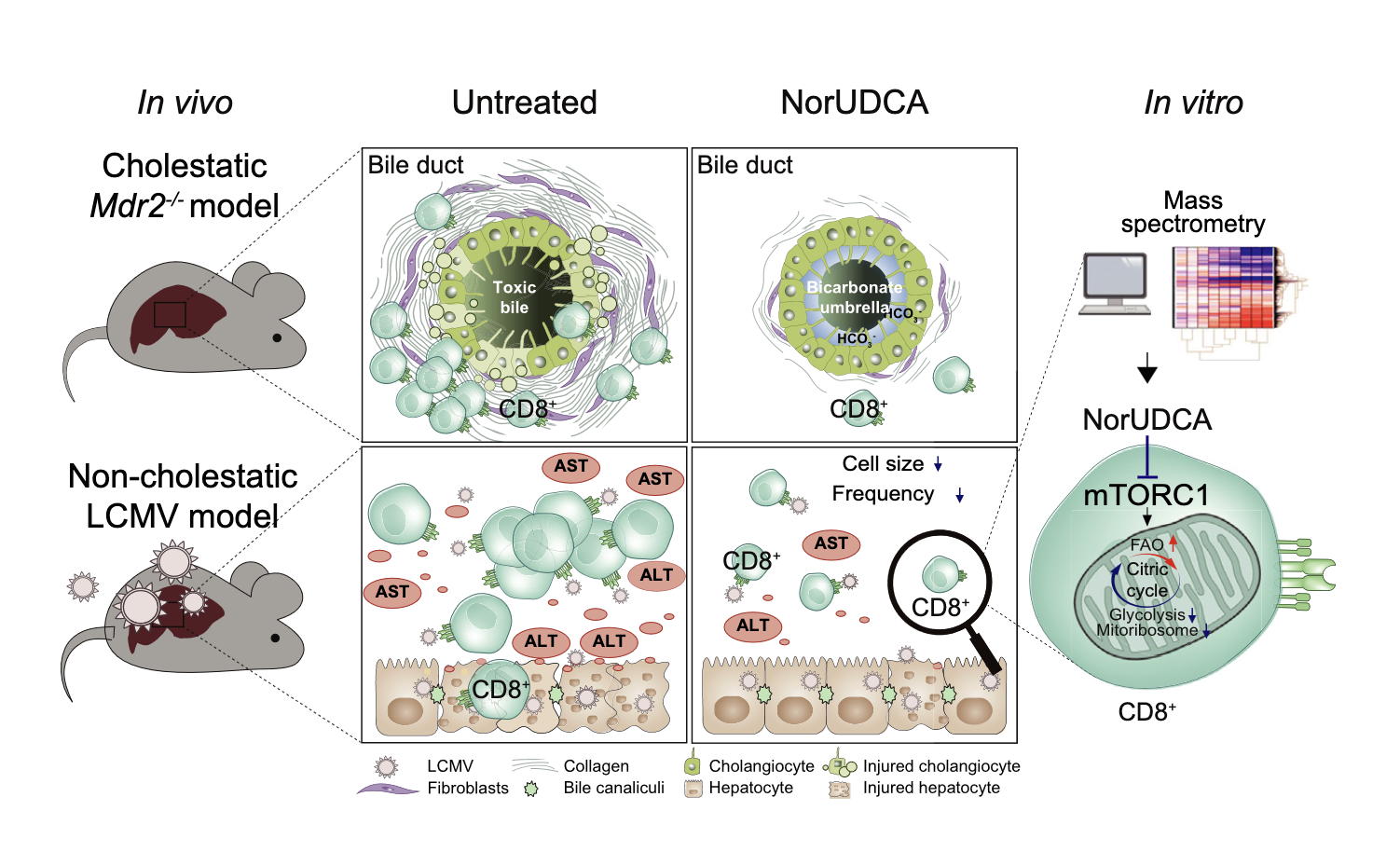
24-Norursodeoxycholic acid reshapes immunometabolism in CD8+ T cells and alleviates hepatic inflammation
Zhu C, Boucheron, N et al … and Ellmeier W, Trauner M. (2021). Journal of Hepatology, 75(5):1164-1176.
PubMed link
more information …
24-Norursodeoxycholic acid (NorUDCA) is a novel therapeutic bile acid used to treat immune-mediated cholestatic liver diseases, such as primary sclerosing cholangitis (PSC), where dysregulated T cells including CD8+ T cells contribute to hepatobiliary immunopathology. In this study, we uncovered that NorUDCA has a direct modulatory impact on CD8+ T cells and attenuates excessive CD8+ T cell-driven hepatic immunopathology. These findings are relevant for treatment of immune-mediated liver diseases such as PSC.
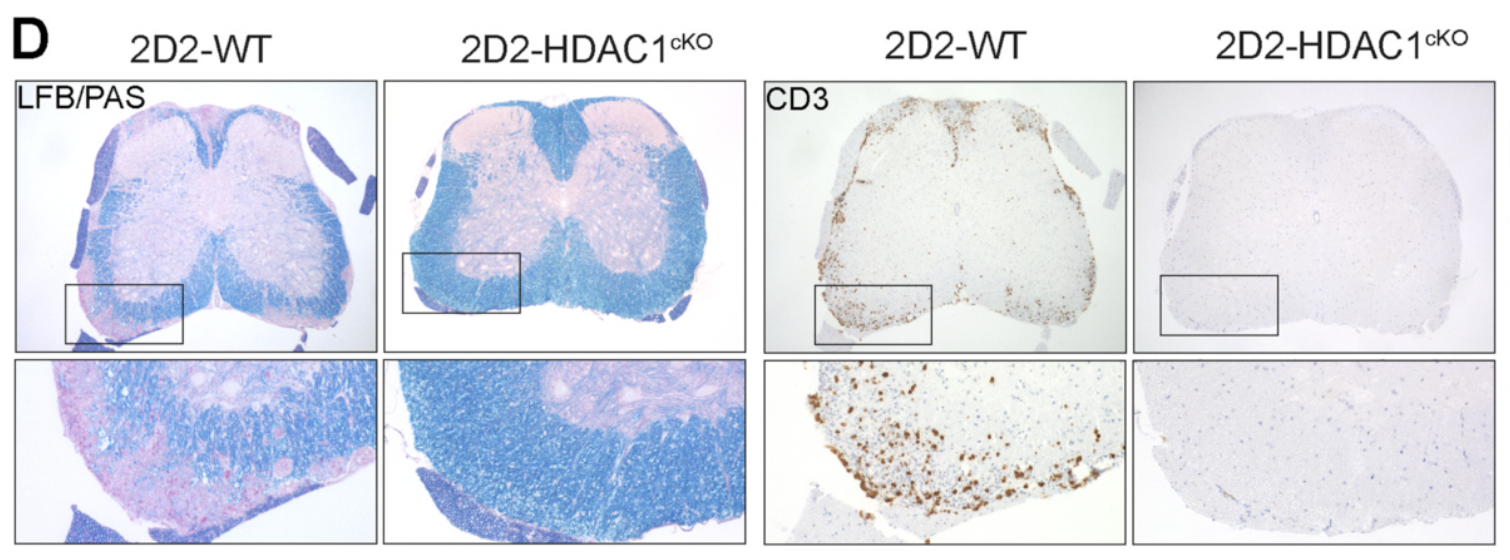
Histone deacetylase 1 controls CD4+ T cell trafficking in autoinflammatory diseases
Hamminger P, et al … and Ellmeier W. (2021). Journal of Autoimmunity, 119:102610.
PubMed link
more information …
CD4+ T cell trafficking is a fundamental property of adaptive immunity. In this study, we uncover a novel role for HDAC1 in controlling effector CD4+ T cell migration, thereby providing mechanistic insight into why a T cell-specific deletion of HDAC1 protects against experimental autoimmune encephalomyelitis (EAE).

